- All Products
- Shop by Brands
- BW Technologies
- BW Technologies
- BW Technologies Monitors
- Models
- BW Clip
- BW Clip4
- GasAlertClip Extreme
- GasAlert Extreme
- GasAlertMicroClip XT
- GasAlertMicroClip XL
- GasAlertMicroClip X3
- GasAlertQuattro
- GasAlertMax XT II
- GasAlertMicro 5 Series
- SamplerPak
- IntelliDox Docking System
- MicroDock II
- BW Ultra
- BW Solo
- Gas Monitors
- Gas Monitor Manufacturers
- BW Technologies
- Bacharach
- Biosystems
- Crowcon
- Gas Clip Technologies
- GfG Instruments
- GMI/Detcon
- Honeywell Analytics
- Industrial Scientific
- MSA
- RAE Instruments
- RKI Instruments
- Calibration Gas
- Combustion Analyzers
- Water Quality
- Reliability Instruments
- Refractometers
- HVAC
- Weather
- Temperature
- Laboratory
- Sound
- IAQ
- Electrical
- All Categories
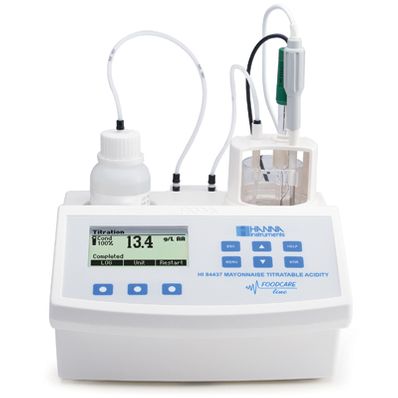
Hanna Titratable Acidity Mini Titrator and pH Meter for Mayonnaise, 230V - HI84437-02
Item #:
HI84437-02
Brand:
Hanna Instruments
Share this product:
HI84437-02 Titratable Acidity Mini Titrator and pH Meter for Mayonnaise, 230V by Hanna Instruments
The HI 84437 is an easy to use automatic mini titrator and pH meter designed for the rapid and accurate analysis of titratable acidity in mayonnaise. By eliminating subjective factors including color indicators, errors in mathematical calculations or erratic titrant additions from the measurement, the HI 84437 makes titratable acidity analysis precise. This instrument will quickly become a valuable tool for mayonnaise analysis.
A clear and intuitive user interface allows users to navigate the HI 84437’s menus and functions quickly. A HELP key located on the keypad aids in set-up, calibration status and troubleshooting.
Simply weigh the sample, dilute with water and press start. The HI 84437 automatically stirs the sample, starts pumps operation and titrates the sample to the endpoint.
The instrument employs a powerful and effective built-in algorithm to analyze the pH response to determine the exact pH endpoint, then uses this to make the necessary calculations.
Titratable acidity determination is instantaneously displayed in the selected measurement unit on the display. The instrument is then immediately ready for the next analysis .
The HI 84437 employs a simple peristaltic pump to ensure the best accuracy and repeatability in measurements. To ensure the most consistent results, pump calibrations can be performed with the provided HANNA standard.
- No filtering, just mix and titrate
- Log on demand up to 100 total samples
- GLP features
- Eliminates subjective factors
- Can measure in % or g/L acetic acid
- Three point calibration
- Automatic pH temperature compensation
- Automatic “anytime” help
- Intuitive user interface
Order Information:
HI 84437-01 (115V) and HI 84437-02 (230V) are supplied with HI 1131B pH electrode, HI 7662-M temperature probe, HI 84437-50 titrant solution (100 mL), HI 84437-55 pump calibration solution (100 mL), HI 7004M pH 4.01 buffer solution (230 mL), HI 7007M pH 7.01 buffer solution (230 mL), HI 70082M pH 8.20 buffer solution (230 mL), 100 mL beakers (2), tube set with dispensing tip, medium magnetic stir bars (2), 12 VDC adapter and instruction manual.
| Titrator Titratable Acidity Range | g/100 g as acetic acid : 0.16 - 1.60% AA g/L (ppt) as acetic acid : 1.6 - 16.0 g/L (ppt) AA |
| Titrator Resolution | 0.01% AA; 0.1 g/L (ppt) AA |
| Titrator Accuracy (@25ºC/77ºF) | 5% of reading |
| Titrator Titration Method | acid-base titration |
| Titrator Pump Debit | 0.5 mL/min |
| Titrator Stirring Speed | 700 rpm |
| Titrator Logging Data | up to 50 samples |
| pH Meter Range | -2.0 to 16.0 pH / -2.00 to 16.00 pH |
| pH Meter Resolution | 0.1 pH / 0.01 pH |
| pH Meter Accuracy (@25ºC/77ºF) | ±0.01 pH |
| pH Meter Calibration | one, two or three calibration points; 3 available buffers (4.01; 7.01; 8.20) |
| pH Meter Temperature Compensation | manual or automatic from -20 to 120°C (-4 to 248°F) |
| pH Meter Logging Data | up to 50 samples |
| Temperature Range | -20.0 to 120.0°C (-4.0 to 248.0°F) |
| Temperature Resolution | 0.1°C |
| Temperature Accuracy (@25ºC/77ºF) | ±0.4°C without probe error |
| Electrodes | HI 1131B glass body pH electrode with BNC connector and 1 m (3.3’) cable |
| Temperature Probe | HI 7662-M stainless steel temperature probe with 1 m (3.3’) cable (included) |
| Environment | 0 to 50°C (32 to 122°F); max 95% RH non-condensing |
| Power Supply | 12 VDC adapter (included) |
| Dimensions | 208 x 214 x 163 mm (8.2 x 8.4 x 6.4”) (with beaker) |
| Weight | 2200 g (77 oz.) |